Abstract
Introduction: Tyrosine kinase inhibitors (TKI) have significantly improved treatment outcomes for patients with newly diagnosed chronic myelogenous leukemia (CML) in chronic phase (CP). With the introduction of 2nd and 3rd generation TKI, the options in the management of CML have increased. In clinical trials, 25%-50% of patients do not remain on initial treatment due to intolerance or inefficacy. Information outside of clinical trials is limited.
Aim: The aim of this study is to analyze the use of different TKI in first-line treatment and the frequency of TKI switches in CML in real world.
Methods: Patients with newly diagnosed CML between 1.1.2013 and 31.12.2019 were registered and followed annually up to 31.12.2021. Primary endpoint was the time to and reason for switching of any first-line treatment including treatment free remission (TFR)attempt. Further endpoints were progression-free survival (PFS), overall survival (OS), cytogenetic and molecular response. Analyses were performed using Kaplan-Meier estimates with SPSS.
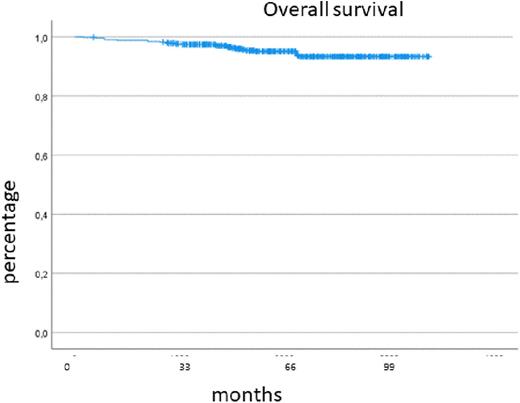
Results: 540 adult patients have been registered from 91 centers, 512 were evaluable. 281 patients (55%), were treated by resident oncologists, 231 patients (45%) in hospitals including 82 patients (16%) in university hospitals. 271 patients (53%) were male. At diagnosis, median age was 59 (range 16-100) years. The median follow-up was 67 (3-108) months. Information on first-line TKI treatment was available for 480 patients: 203 patients (42%) were treated with imatinib, 137 (29%) with nilotinib and 140 (29%) with dasatinib. TKI switch was reported in 155 cases (31%) after a median of 11 (0-68) months. Reasons for switching was failure in 49 patients (32%), intolerance in 88 patients (57%), and 18 patients (11%) switched TKI because of other reasons (study inclusion, desire to have children, patient request, unknown). 33 patients (7%) switched TKI more than once. 13 (8,4%) of the 155 patients with TKI-switch died during follow-up, whereas only 16 of 325 (4,9%) patients without TKI-switch. OS of patients with TKI-switch was 90%, of patients without TKI-switch 94% after 6 years. TKI stop for more than one month has been reported in 118 patients (24%) due to different reasons (attempt for TFR, intolerance, patient wish, pregnancy). 61 patients (12%) started a TFR attempt after a median of 46 (10-96) months of TKI treatment. Discontinuation criteria were not always met. Only 10 patients (6,5%) with TKI-switch started a TFR attempt compared to 51 patients (16%) who had only been treated with one TKI.
501 patients were in CP at diagnosis, 11 patients in accelerated phase (AP) and 2 patients in blast crisis (BC). Progression occurred in 9 patients (BC n=6, AP n=3) after a median time of 12 (9-22) months. Overall, AP and BC were diagnosed in 22 patients (2,3%). Of the total cohort, 29 patients died, OS was 93% after 6 years.
An evaluable cytogenetic examination was carried out in 291 patients (57%) at diagnosis or in the course of disease. No statements can be made about achieving of complete cytogenetic remission as follow up results were only available in 15% and monitoring was carried out using RQ-PCR in most patients.
A major molecular response (MMR, BCR::ABL1 <0.1%) was reached by 422 patients (85%), a deep molecular response (defined as BCR::ABL1 ≤0.01) by 365 patients (74%) within the follow-up period. Cumulative incidence calculation and study of patients who lost their MMR are ongoing. There is no information about the molecular status of 18 patients.
Conclusion: Results of the CML VI-registry provided information on treatment but also on baseline characteristics and diagnostic parameters of CML patients in real world. The median age of the patients at diagnosis was higher than in other clinical trials. In routine care, data demonstrated high switching rates of first-line treatment and frequent TFR attempts without meeting the guideline criteria. OS rates were as high as in clinical trials and confirm normal life expectation of CML patients. MMR was reached in 85% and DMR in 74% in this population. Progression is rare. In conclusion, the CML-registry offers importing data confirming a good outcome of response and survival for CML patients outside of clinical trials.
Disclosures
Gattermann:Takeda: Research Funding; Celgene: Honoraria; BMS: Honoraria; Novartis: Honoraria. Brummendorf:Novartis: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Pfizer Inc: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Merck: Consultancy, Other: Travel, Accommodation, Expenses; Janssen: Consultancy, Other: Travel, Accommodation, Expenses. le Coutre:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Burchert:Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AOP Health: Honoraria, Research Funding. Hochhaus:Incyte: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Bristol Myers Squibb: Research Funding. Saussele:Incyte: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal